Enzymes were first discovered by Edward Buchner in 1897. He found that even an extract from the yeat cells could ferment glucose. He named those factors in the yeast responsible for fementation as enzymes. The term enzymes literally means 'in yeast' and is now used as a common name for all biological catalysts.
Almost
all enzymes are proteins. There are some nucleic acids that behave like
enzymes. These are called ribozymes. One can depict an enzyme
by a line diagram. An enzyme like any protein has a primary structure, i.e.,
amino acid sequence of the protein. An enzyme like any protein has the
secondary and the tertiary structure. When you look at a tertiary structure you
will notice that the backbone of the protein chain folds upon itself, the chain
criss-crosses itself and hence, many crevices or pockets are made. One such
pocket is the ‘active site’.
An active site of an enzyme is a crevice or pocket
into which the substrate fits. Thus enzymes, through their active site,
catalyse reactions at a high rate. Enzyme catalysts differ from inorganic
catalysts in many ways, but one major difference needs mention. Inorganic
catalysts work efficiently at high temperatures and high pressures, while
enzymes get damaged at high temperatures (say above 40°C). However, enzymes
isolated from organisms who normally live under extremely high temperatures
(e.g., hot vents and sulphur springs), are stable and retain their catalytic
power even at high temperatures (upto 80°-90°C ( Example: Taq polymerase).
Thermal stability is thus an important quality of such enzymes isolated from
thermophilic organisms.
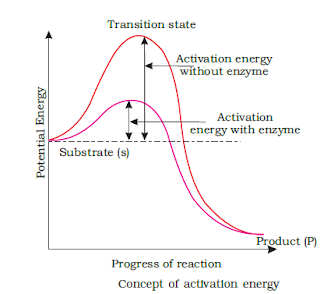
The molecules of various substances have certain energy levels. The molecule that take part in chemical reactions are at high energy levels. The energy required to reach the molecules at high levels is called the activation energy.
The rate of an enzyme related reaction may be changed by concentration, pH, Temperature.
Classification and Nomenclature of Enzymes
Co enzymes: Enzymes are composed of one or several polypeptide
chains. However, there are a number of cases in which non-protein constituents
called cofactors are bound to the the enzyme to make the enzyme catalytically
active. In these instances, the protein portion of the enzymes is called
the apoenzyme. Three kinds of cofactors may be identified:
prosthetic groups, co-enzymes and metal ions.
Prosthetic groups are organic compounds and are distinguished from
other cofactors in that they are tightly bound to the apoenzyme. For
example, in peroxidase and catalase, which catalyze the breakdown of
hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a
part of the active site of the enzyme.
The apoenzyme and prosthetic group together are called holoenzyme
Co-enzymes are also organic compounds but their association with the apoenzyme is only transient, usually occurring during the course of catalysis. Furthermore, co-enzymes serve as co-factors in a number of different enzyme catalyzed reactions. The essential chemical components of many coenzymes are vitamins, e.g., coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin.
A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site and at the same time form one or more cordination bonds with the substrate, e.g., zinc is a cofactor for the proteolytic enzyme carboxypeptidase. Catalytic activity is lost when the co-factor is removed from the enzyme which testifies that they play a crucial role in the catalytic activity of the enzyme.
Summary: Enzymes are proteins which catalyse biochemical reactions in the cells. Ribozymes are nucleic acids with catalytic power. Proteinaceous enzymes exhibit substrate specificity, require optimum temperature and pH for maximal activity. They are denatured at high temperatures. Enzymes lower activation energy of reactions and enhance greatly the rate of the reactions. Nucleic acids carry hereditary information and are passed on from parental generation to progeny.
