Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
Proteins can be purified based on characteristics such as size and shape, total charge, hydrophobic groups present on the surface, and binding capacity with the stationary phase.
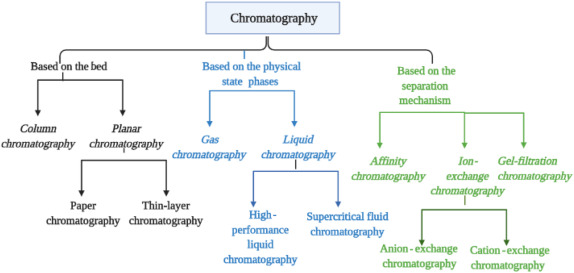
Four separation techniques based on molecular characteristics and interaction type use mechanisms of ion exchange, surface adsorption, partition, and size exclusion.
Other chromatography techniques are based on the stationary bed, including column, thin layer, and paper chromatography.
Based on this approach three components form the basis of the chromatography technique.
Stationary phase: This phase is always composed of a “solid” phase or “a layer of a liquid adsorbed on the surface a solid support”.
Mobile phase: This phase is always composed of “liquid” or a “gaseous component.”
Separated molecules
Stationary phase in chromatography, is a solid phase or a liquid phase coated on the surface of a solid phase. Mobile phase flowing over the stationary phase is a gaseous or liquid phase. If mobile phase is liquid it is termed as liquid chromatography (LC), and if it is gas then it is called gas chromatography (GC). Gas chromatography is applied for gases, and mixtures of volatile liquids, and solid material. Liquid chromatography is used especially for thermal unstable, and non-volatile samples
The purpose of applying chromatography which is used as a method of quantitative analysis apart from its separation, is to achieve a satisfactory separation within a suitable time interval. Various chromatography methods have been developed to that end. Some of them include column chromatography, thin-layer chromatography (TLC), paper chromatography, gas chromatography, ion exchange chromatography, gel permeation chromatography, high-pressure liquid chromatography, and affinity chromatography
Types of chromatography
- Column chromatography (Adsorption Chromatography)
- Ion-exchange chromatography
- Gel-permeation (molecular sieve) chromatography
- Affinity chromatography
- Paper chromatography
- Thin-layer chromatography
- Gas -Liquid chromatography
- High-pressure liquid chromatography (HPLC)
The type of interaction between stationary phase, mobile phase, and substances contained in the mixture is the basic component effective on separation of molecules from each other. Chromatography methods based on partition are very effective on separation, and identification of small molecules as amino acids, carbohydrates, and fatty acids.
Affinity chromatographies (ie. ion-exchange chromatography) are more effective in the separation of macromolecules as nucleic acids, and proteins.
Paper chromatography is used in the separation of proteins, and in studies related to protein synthesis;
Gas-liquid chromatography is utilized in the separation of alcohol, esther, lipid, and amino groups, and observation of enzymatic interactions,
Molecular-sieve chromatography is employed especially for the determination of molecular weights of proteins.
Column chromatography is one of the most common methods of protein purification.